
If our defective medical device attorney believes you may have a case, we will obtain a medical review of your records to determine if you are eligible to bring a defective product claim for injuries from an INFUSE bone graft. Our firm has a long track record of successful financial recovery for injured people in California, Nevada and across the U.S.Ĭontact us online today – your consultation is free. The San Francisco law firm of Walkup, Melodia, Kelly & Schoenberger represents people who have been injured by defective medical devices and dangerous drugs. If you are experiencing pain or have other side effects from your INFUSE bone graft, we can help. Numerous patients who have received an INFUSE bone graft are now experiencing serious side effects and have become injured due to the graft. Likewise, the FDA has not approved INFUSE for spinal surgery involving the upper back and neck (the cervical spine). INFUSE was not approved (and still is not approved) for other types of spinal surgeries, such as posterior-approach lumbar interbody fusion surgeries (where the surgeon operates through the back).In this surgery, the surgeon operates on the spine through an incision in the abdomen. Food and Drug Administration (FDA) approved INFUSE for only one type of spine surgery: anterior approach lumbar interbody fusion (ALIF). It is used in place of the patient’s own bone in spinal fusion surgery. INFUSE (bone morphogenetic protein-2) stimulates bone growth in the space between vertebrae. Medtronic, a medical device manufacturer, promoted and sold the INFUSE bone graft product for the purpose of strengthening the lower spine by fusing two adjacent vertebrae together. Contact us online, or call 1-800-BAD-DRUG today.Millions of Americans suffer back pain from degenerative disk disease and other conditions for which they receive physical therapy, pain medication and, in some cases, spinal surgery. Our legal professionals are ready to answer your questions and help you determine whether you may have a claim against the makers of the InFuse Bone Graft device for your medical complications. If you or a loved one has suffered because of off-label use of the Infuse Bone Graft device, it is important to seek legal advice as quickly as possible.
#Infuse bone skin#
According to the report, people who received Infuse may be at higher risk for all types of cancers, including pancreatic, breast, stomach, ovarian, lung, prostate, lymphoma and skin cancers.
#Infuse bone trial#
InFuse Bone Graft Cancer RiskĪ recent clinical trial has raised concerns about a possible link between InFuse Bone Graft and an increased risk of cancer. Many of the patients required immediate treatment, including tracheotomies, feeding tubes and additional surgical procedures. Patients reported constriction of the airway and nerves in the neck, which in turn caused difficulty breathing, swallowing and speaking. Following reports of such medical complications, the FDA issued a warning in 2008, advising that the risks of using the device to treat the cervical spine had not been studied and “ that the safety and effectiveness of in the cervical spine have not been demonstrated and these products are not approved by FDA for this use.” Now, many of those patients who received such off-label treatment with the InFuse device are coming forward with verifiable, serious, sometimes life-threatening problems. It has been estimated that Medtronic, the maker of the device, has generated revenue in excess of $3 billion for sales related to such off-label use. However, the parent company, Medtronic is being accused of encouraging doctors and other medical providers to use the InFuse Bone Graft devices to repair cervical spine (neck) injuries when that was never the intended use of the product. Food & Drug Administration (FDA) approved the Medtronic InFuse Bone Graft for use in the lower back and for specific dental procedures. It has been approved as a replacement for damaged spinal disks in the lower back (lumbar spine).

The cage structure allows natural bone material to grow, develop and strengthen at the surgery site. The device was developed for use as an alternative to natural bone grafts, wherein bone is taken from one part of the patient’s body and transferred to another. Male sterility, retrograde ejaculation, or other uro-genital injuries.Chronic radiating pain in the legs or arms.Uncontrolled bone growth at or near the site of the surgery.Difficulty Breathing, Swallowing or Speaking.When used as approved or off-label, patients report these and other serious side effects, including: The swelling can occur immediately or there may be a delay.
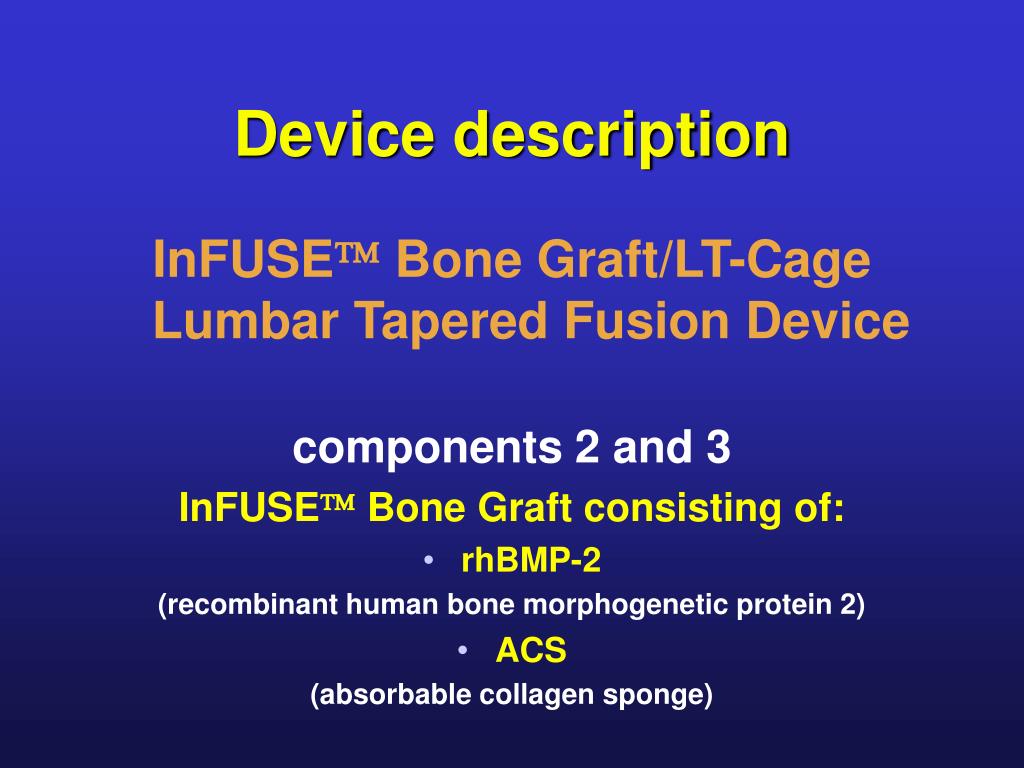
Many patients, however, are allergic to the protein and develop severe and rapid swelling of the mouth, nose and throat. InFuse also contains a synthetically engineered protein that promotes bone growth in and around the cage mesh. The time you have to pursue a claim is limited.


 0 kommentar(er)
0 kommentar(er)
